In my opinion, the starting point of all human existence is conscious experience…ie. the direct first person awareness of my own mental states.
Upon reflecting on these mental states there are some I find enjoyable and others that I do not.
Thus, it seems to me that there is one thing we are directly after…
Feelings
The ultimate things ALL humans DIRECTLY desire are enjoyable feelings.
Get the best feelings by inputting (into your space) the best things (water/protons, photons, electrons, oxygen, etc.) in the best amounts at the best times in the best order.
Here’s how…
Space
Space is defined directly and intuitively by taking a step to the left then a step back to the right (x), jumping up and then coming back down (y), and by taking a step forward then a step back (z).
Space is modeled as Cartesian x, y, and z coordinates for our purposes.
Time
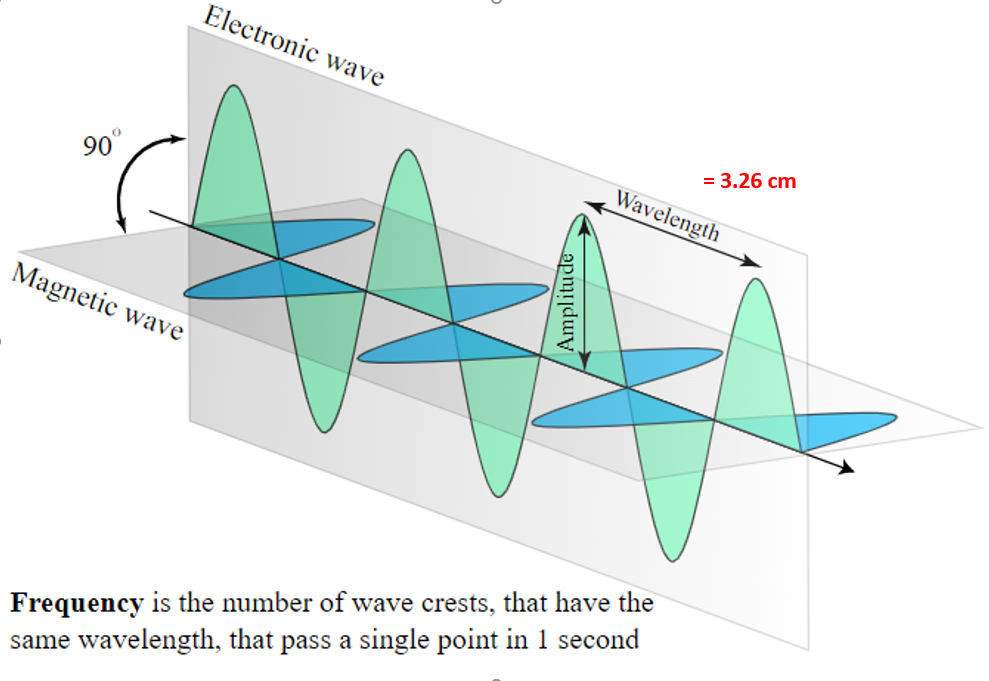
Time is defined as an event or events happening at some location (x,y,z) in 3D space (ie. a clock tick, a train arrival, an Earth Rotation, a Sun Orbit, 9,197,631,770 crests of a 3.26 cm microwave wavelength photon of light traveling through a vacuum and hitting a detector, etc.)


Waking
Space: your bed
Time: 6am
Substance: Wake up at the same time ALL days (Earth Rotations) and stay awake for 16 hours per day until your death date.
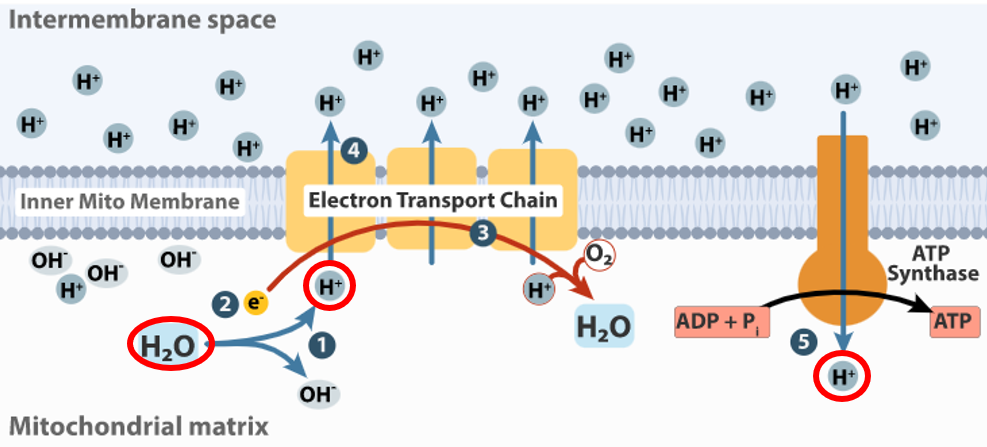
Water = Protons
Space: your mouth, stomach, mitochondrial matrix
Time: 6am
Substance: Protons = water = Drink 13 cups (104 fluid ounces) per Earth Rotation (4 cups (32 fluid ounces) of water upon waking at 6am and 9 cups (72 fluid ounces) for the 15 hours of remaining wakefulness at .6 cups (4.8 fluid ounces) per hour.

Light = Photons = Master Controller of ALL Mitochondria
Space: Retina (Rods, Intrinsically Photosensitive Retinal Ganglion Cells, retinal)
Time: 6am to 6:30am
Substance: Photons = Bright Light Therapy = get 30 minutes of 10,000 lux bright light from 12 inches away using this affordable $39.99 device: Verilux HappyLight

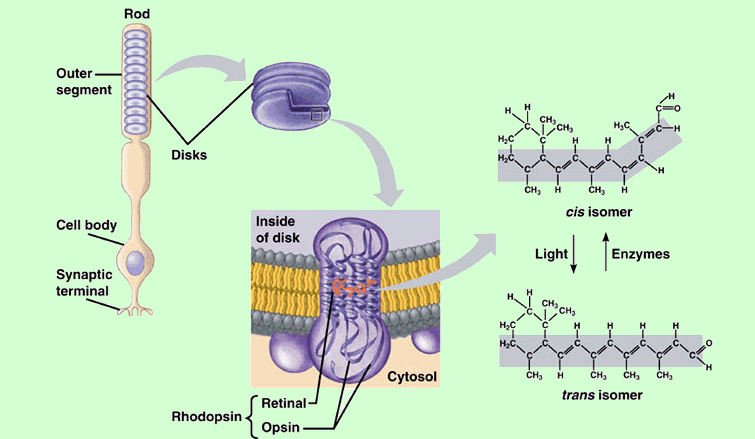
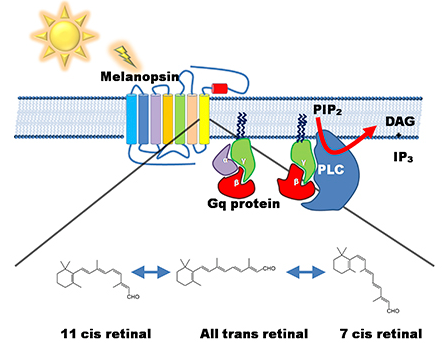
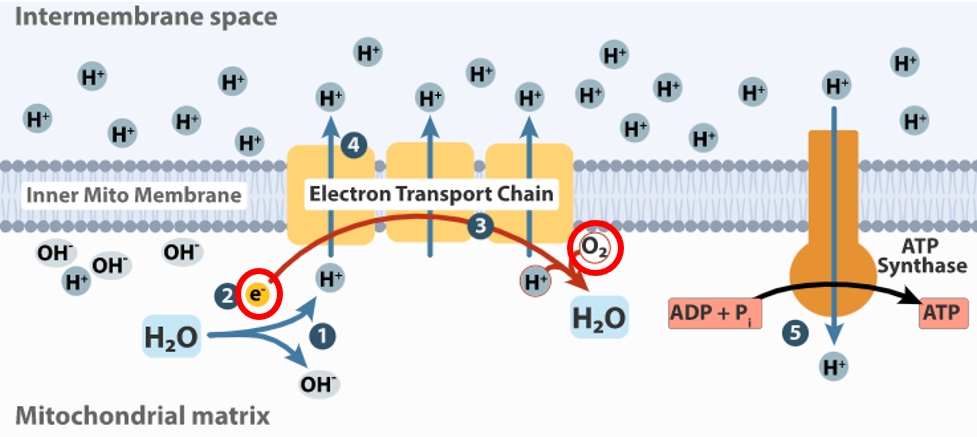
Oxygen = Electron Acceptor
Space: Your nose, lungs, mitochondrial matrix
Time: 6:30am to 7:00am
Substance: Walk for 30 minutes at the same time each day for ALL days until your death date to enhance oxygen absorption and delivery and thereby enhance electron acceptance and total energy levels.
Food = Electrons
Space: mouth, digestive tract, mitochondrial matrix
Time: 7:00am to 7:30am; 11:00am to 11:30am; 2:30-3:00pm (ideal 8 hour feeding window given 6am wake up time; adjust to 10 or 12 hour feeding window if necessary)
Substance: Food = Calories = electrons; Go to the free site Cronometer and enter your age, height, weight, and activity level to determine the number of kilocalories to eat per Earth Rotation (example below = 2529 kcal). Eat three meals at the same time ALL days with 50% (1265 kcal) of calories at breakfast, 30% (759 kcal) of calories at lunch, and 20% (506 kcal) of calories at dinner.

Quick Tips For Future Discussion as we work our way through each element of space, time, the Standard Model of particle physics, and the periodic table:
Take 1.15 mg of Lithium Orotate per day at breakfast. Use this Pill Cutter pill cutter to cut each pill into fourths.
Take 3mg of Boron per day at breakfast.
Lostfalco’s Dynamic Synbiotic Duo: Drink 8 fluid ounces total of Kombucha and 10 grams total of Dextrin (in water) at the beginning of each meal with alternating sips between Kombucha and Dextrin.
Kombucha is filled with healthy bacteria and dextrin feeds those bacteria immediately in the stomach so you can get instant effects instead of waiting 2 to 5 hours for the dextrin to move through the stomach and enter the small intestine.
Take 4 teaspoons of Extra Virgin Olive Oil at the beginning of each meal to complete the Holy Trinity of Satiety (Kombucha + Dextrin + Olive Oil).
Enhance satiety even further by eating protein right after your (kombucha + dextrin + olive oil) and carbohydrates last. The best protein source is EPA and DHA rich Mackerel.
Scientific Studies
INSULIN
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3478548/
Diurnal Pattern to Insulin Secretion and Insulin Action in Healthy Individuals
Abstract
Evaluation of the existence of a diurnal pattern of glucose tolerance after mixed meals is important to inform a closed-loop system of treatment for insulin requiring diabetes. We studied 20 healthy volunteers with normal fasting glucose (4.8 ± 0.1 mmol/L) and HbA1c (5.2 ± 0.0%) to determine such a pattern in nondiabetic individuals. Identical mixed meals were ingested during breakfast, lunch, or dinner at 0700, 1300, and 1900 h in randomized Latin square order on 3 consecutive days. Physical activity was the same on all days. Postprandial glucose turnover was measured using the triple tracer technique. Postprandial glucose excursion was significantly lower (P < 0.01) at breakfast than lunch and dinner. β-Cell responsivity to glucose and disposition index was higher (P < 0.01) at breakfast than lunch and dinner. Hepatic insulin extraction was lower (P < 0.01) at breakfast than dinner. Although meal glucose appearance did not differ between meals, suppression of endogenous glucose production tended to be lower (P < 0.01) and insulin sensitivity tended to be higher (P < 0.01) at breakfast than at lunch or dinner. Our results suggest a diurnal pattern to glucose tolerance in healthy humans, and if present in type 1 diabetes, it will need to be incorporated into artificial pancreas systems.
https://journals.sagepub.com/doi/epdf/10.1177/000456329903600407
Diurnal variations in peripheral insulin resistance and plasma non-esterified fatty acid concentrations: a possible link?
Abstract
Glucose tolerance becomes impaired towards the evening. Increased peripheral insulin resistance may be responsible, at least in part, for this effect. The mechanism for the diurnal variation in insulin sensitivity is undefined. It is, however, possible that variations in non-esterified fatty acids (NEFA) could contribute to this variation because NEFA have been implicated in the pathogenesis of insulin resistance. Therefore, we have investigated insulin sensitivity and plasma NEFA responses to insulin at 0830 h and 2030 h in nine healthy men by measuring arterialized plasma glucose and venous plasma NEFA concentrations during a short insulin tolerance test. The studies were standardized for a period of fasting, pre-test meal and exercise. Insulin sensitivity measured KITT was greater (P < 0.05) in the morning [(20 +/- 7) x 10(-3) mmol/L/min] than in the evening [(11.6 +/- 2) x 10(-3) mmol/L/min]. Fasting NEFA levels were lower (P < 0.01) in the morning (373 +/- 84 mumol/L) than in the evening (913 +/- 122 mumol/L). Following insulin, NEFA fell more slowly (P < 0.01) in the morning (149 +/- 26 mumol/L/15 min) than in the evening (491 +/- 91 mumol/L/15 min). These results confirm diurnal variations in insulin sensitivity and plasma NEFA concentrations irrespective of feeding and exercise. We speculate that the relatively elevated plasma NEFA levels in the evening are the cause rather than the consequence of increased insulin resistance at this time.
https://diabetesjournals.org/diabetes/article-pdf/41/6/750/359106/41-6-750.pdf
Diurnal variation in glucose tolerance. Cyclic suppression of insulin action and insulin secretion in normal-weight, but not obese, subjects
Abstract
The relative roles of insulin sensitivity, insulin secretion, and glucose effectiveness to the diurnal rhythm of glucose tolerance were examined in normal-weight (n = 12) and obese (n = 11) subjects. Two frequently sampled intravenous glucose tolerance tests were performed in each subject at 0800 on one occasion and 1800 on a separate day. Tests were preceded by identical fasts of 10-12 h. In nonobese subjects, glucose tolerance, expressed as the 10- to 16-min KG value (KGs), was much reduced in the evening (AM 2.98 +/- 0.45, PM 1.86 +/- 0.33 min-1, P less than 0.002). In the obese subjects, tolerance was lower in the morning than normal-weight subjects (2.19 +/- 0.31 min-1), but unlike in nonobese subjects, tolerance was not significantly reduced during the day (1.90 +/- 0.18 min-1, P greater than 0.40). The reduction in glucose tolerance in the normal-weight subjects was caused by diminished insulin sensitivity (parameter S1, AM 15.4 +/- 2.9, PM 10.2 +/- 1.9 x 10(-5) min-1/pM, P less than 0.01) and reduced beta-cell responsivity to glucose. The evening decrease in the latter was reflected both in first-phase plasma insulin (AM 2466 +/- 441, PM 1825 +/- 381 pM/10 min, P less than 0.05) and the potentiation slope (AM 462 +/- 68, PM 267 +/- 35 pM/mM, P less than 0.01). In contrast, consistent with no diurnal variation in glucose tolerance, obese subjects exhibited no decline in insulin sensitivity in the evening (AM 3.6 +/- 0.7, PM 4.9 +/- 1.0 x 10(-5) min-1/pM).(ABSTRACT TRUNCATED AT 250 WORDS)
LITHIUM
https://pubmed.ncbi.nlm.nih.gov/11838882/
Lithium: occurrence, dietary intakes, nutritional essentiality
Abstract
Lithium is found in variable amounts in foods; primary food sources are grains and vegetables; in some areas, the drinking water also provides significant amounts of the element. Human dietary lithium intakes depend on location and the type of foods consumed and vary over a wide range. Traces of lithium were detected in human organs and fetal tissues already in the late 19th century, leading to early suggestions as to possible specific functions in the organism. However, it took another century until evidence for the essentiality of lithium became available. In studies conducted from the 1970s to the 1990s, rats and goats maintained on low-lithium rations were shown to exhibit higher mortalities as well as reproductive and behavioral abnormalities. In humans defined lithium deficiency diseases have not been characterized, but low lithium intakes from water supplies were associated with increased rates of suicides, homicides and the arrest rates for drug use and other crimes. Lithium appears to play an especially important role during the early fetal development as evidenced by the high lithium contents of the embryo during the early gestational period. The biochemical mechanisms of action of lithium appear to be multifactorial and are intercorrelated with the functions of several enzymes, hormones and vitamins, as well as with growth and transforming factors. The available experimental evidence now appears to be sufficient to accept lithium as essential; a provisional RDA for a 70 kg adult of 1,000 microg/day is suggested.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6443601/
Is Lithium a Micronutrient? From Biological Activity and Epidemiological Observation to Food Fortification
Abstract
Lithium compounds have been widely used in psychopharmacology, particularly in the treatment of bipolar disorder. Their normothymic and neuroprotective properties when used at high doses have been well established. However, a number of observations suggest that environmentally relevant lithium doses may also exert beneficial health effects, leading to a decrease in the rate of suicides and levels of violence. Despite the fact that this element is not officially considered to be a micronutrient, some authors have suggested provisional recommended intakes set at 1000 μg/day for a 70-kg adult (14.3 μg/kg body weight). The present paper reviews the biological action of lithium, its bioavailability and metabolism, and content in different foodstuffs and water. It also assesses epidemiological data on potential correlations between lithium intake and suicide rate as well as examines the concept of fortifying food with this element as a strategy in the primary prevention of mood disorders and pre-suicidal syndrome.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5751281/
An Oldie but Goodie: Lithium in the Treatment of Bipolar Disorder through Neuroprotective and Neurotrophic Mechanisms
Abstract
Lithium has been used for the treatment of bipolar disorder (BD) for the last sixty or more years, and recent studies with more reliable designs and updated guidelines have recommended lithium to be the treatment of choice for acute manic, mixed and depressive episodes of BD, along with long-term prophylaxis. Lithium’s specific mechanism of action in mood regulation is progressively being clarified, such as the direct inhibition on glycogen synthase kinase 3β, and its various effects on neurotrophic factors, neurotransmitters, oxidative metabolism, apoptosis, second messenger systems, and biological systems are also being revealed. Furthermore, lithium has been proposed to exert its treatment effects through mechanisms associated with neuronal plasticity. In this review, we have overviewed the clinical aspects of lithium use for BD, and have focused on the neuroprotective and neurotrophic effects of lithium.
https://ane.pl/linkout.php?pii=7601
Lithium – therapeutic tool endowed with multiple beneficiary effects caused by multiple mechanisms
Abstract
Mood disorders are relatively common serious human diseases for which there is often no ideal pharmacotherapy. Basic characteristic of these diseases is affective disorder shifting the mood of the patient to depression (together with anxiety or not) or towards to euphoria. Available drugs are usually divided into two groups – mood stabilizers, which are used primarily to treat bipolar disorder, and antidepressants for the treatment of unipolar depression. Lithium is still recommended as the first choice for dealing with bipolar disorder. Despite abundant clinical use of mood stabilizing drugs, important questions regarding their mechanism of action remain open. In this paper we present the brief review of rather diversified hypotheses and ideas about mechanisms of genesis of mood disorders and lithium interferences with these pathological states. New data derived from the high-resolution crystallographic studies of allosteric, Na+-binding sites present in G protein coupled receptors are given together with data indicating the similarity between lithium and magnesium cations. In this context, similarities and dissimilarities between the useful “poison” with narrow therapeutic window (Li+) and the bivalent cation acting like cofactor of more than 300 enzymatic reactions (Mg2+) are pointed out together with results indicating enhanced activity of trimeric G proteins in bipolar disorder.
Lithium: Perspectives of nutritional beneficence, dietary intake, biogeochemistry, and biofortification of vegetables and mushrooms
Abstract
Although lithium (Li) is not an essential nutrient for humans, low Li intakes are associated with increased suicide and homicide rates, aggressive behaviors, unipolar/bipolar disorders, acute mania, etc. On the other hand, Li is one of the most effective psychopharmacological agents used for the treatment of these psycho-behavioral disorders. The beneficial normothymic effect of Li could be achieved at lower doses, therefore, modern psychiatry has called to consider Li biofortification of foods to improve its dietary intake. The concept of agronomic biofortification of crops with Li is juvenile and there exist a limited number of studies, mainly focused on vegetables or mushrooms. This review, first of its kind, discusses the nutritional beneficence and dietary intake of Li, its biogeochemistry, and opportunities and challenges in the Li biofortification of food crops. Literature showed that dietary intake of Li in many countries of the world is insufficient, compared to the provisional recommended dietary allowance (RDA) of 1.0 mg day−1 for a 70 kg adult. Lithium contents of soils are widely variable and the metal has high mobility in soils, making it more prone to leaching, and available for plant uptake. Biofortification studies reveal that plants can accumulate significant quantities of Li in their edible tissues without yield loss and quality associated negative effects. At lower application rates, Li tissue concentration could reach to the level that consuming 100–200 g of Li-biofortified fresh vegetables or mushrooms could support its RDA. It seems impossible to enrich the plants with Li to the levels that allow their application in psychiatric treatments, which requires the dosage of 600–1200 mg day−1. However, there is need to refine the methods of Li biofortification strategies to obtains plant specific concentration of Li in edible parts so that consuming a specific amount could provide the proposed dietary intake requirement.
https://www.sciencedirect.com/science/article/abs/pii/S0306987701913751
On the physiological function of lithium from a psychiatric view point
Abstract
Lithium is a naturally occurring alkali metal, which living organisms ingest from dietary sources and which is also present in trace amounts in the human body. In much higher concentrations, lithium is effective as a medication for mania and mood swings including manic depressive disorders. Many studies have shown that a deficiency in ‘endogenous’ lithium, i.e. lithium in food and drinking water, can lead to defects in growth and development in animals and to grave psychopathological problems in humans. It is therefore conceivable that lithium has an essential function in the physiological regulation of mood and that a subgroup of pathological mood disorders cause a bodily requirement for drastically higher concentrations of lithium in compartments. Taking lithium long-term could have a prophylactic effect on this kind of change in bodily requirements.
APPENDIX
White Noise I Used While Writing This Post
40 Hz Binaural Beats I Used While Writing This Post








Leave a Reply
Your email is safe with us.