Ibudilast as a Nootropic
Ibudilast (MN-166 or AV-411) is on my short list of the best nootropics I’ve tried (along with LLLT, intranasal insulin, concentrated oxygen, etc.).
I’ve written previously about how it works and where to buy it here but I love to actually show people the science (that’s why I post so many abstracts!).
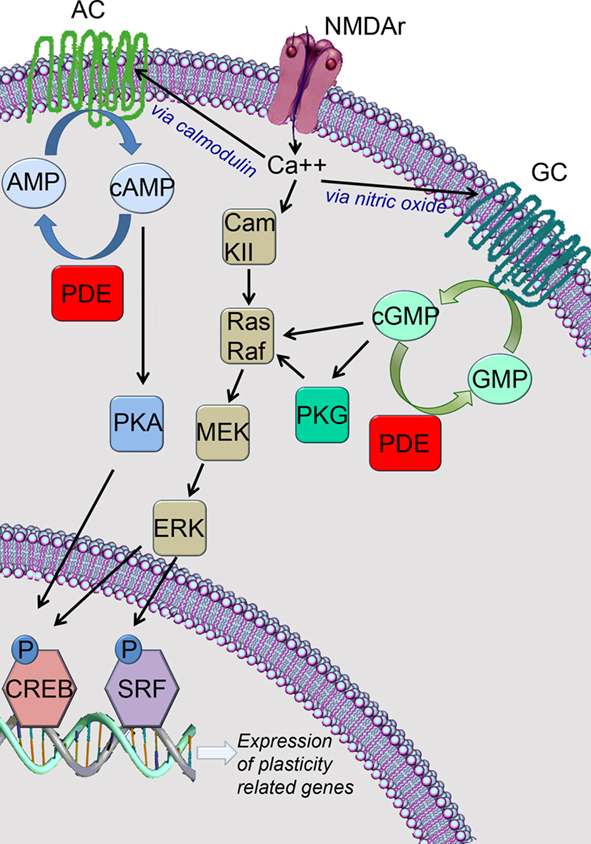
It’s currently the best phosphodiesterase 4 inhibitor available imo which means that it enhances cerebral blood flow, significantly decreases brain inflammation, and should massively increase the expression of genes related to learning and memory (through cAMP/PKA/CREB).
Here’s a nice review article that summarizes the data on phosphodiesterase 4 quite nicely. PDE4 as a target for cognition enhancement.
Check out this short video (watch at 2x speed) to see how this pathway works.
https://www.youtube.com/watch?v=z1QEirj1q7g
So, in the spirit of my previous LLLT and intranasal insulin research posts, I decided to make a post where I compile all of the available human studies on ibudilast.
I’ll keep updating this post regularly as new data comes in.
Read away!
Ibudilast: The Human Studies
https://www.ncbi.nlm.nih.gov/pubmed/27269956
J Clin Psychopharmacol. 2016 Jun 6. [Epub ahead of print]
Safety of Intravenous Methamphetamine Administration During Ibudilast Treatment.
DeYoung DZ1, Heinzerling KG, Swanson AN, Tsuang J, Furst BA, Yi Y, Wu YN, Moody DE, Andrenyak DM, Shoptaw SJ.
Author information
Abstract
BACKGROUND:
Methamphetamine dependence is a significant public health concern without any approved medications for treatment. We evaluated ibudilast, a nonselective phosphodiesterase inhibitor, to assess the safety and tolerability during intravenous methamphetamine administration. We conducted a randomized, double-blind, placebo-controlled, within-subjects crossover clinical trial.
METHODS:
Participants received ibudilast (20 mg twice daily followed by 50 mg twice daily) and placebo, with order determined by randomization, and then underwent intravenous methamphetamine challenges (15 and 30 mg). We monitored cardiovascular effects, methamphetamine pharmacokinetics, and reported adverse events.
RESULTS:
Ibudilast treatment had similar rates of adverse events compared with placebo, and there was no significant augmentation of cardiovascular effects of methamphetamine. Pharmacokinetic analysis revealed no clinically significant change in maximum concentration or half-life of methamphetamine with ibudilast.
CONCLUSIONS:
Methamphetamine administration during ibudilast treatment was well tolerated without additive cardiovascular effects or serious adverse events, providing initial safety data to pursue ibudilast’s effectiveness for the treatment of methamphetamine dependence.
https://www.ncbi.nlm.nih.gov/pubmed/26993372
Drug Alcohol Depend. 2016 May 1;162:245-50. doi: 10.1016/j.drugalcdep.2016.02.036. Epub 2016 Mar 3.
Ibudilast attenuates subjective effects of methamphetamine in a placebo-controlled inpatient study.
Worley MJ1, Heinzerling KG2, Roche DJ3, Shoptaw S2.
Author information
Abstract
BACKGROUND:
Despite numerous clinical trials no efficacious medications for methamphetamine (MA) have been identified. Neuroinflammation, which has a role in MA-related reward and neurodegeneration, is a novel MA pharmacotherapy target. Ibudilast inhibits activation of microglia and pro-inflammatory cytokines and has reduced MA self-administration in preclinical research. This study examined whether ibudilast would reduce subjective effects of MA in humans.
METHODS:
Adult, non-treatment seeking, MA-dependent volunteers (N=11) received oral placebo, moderate ibudilast (40mg), and high-dose ibudilast (100mg) via twice-daily dosing for 7days each in an inpatient setting. Following infusions of saline, MA 15mg, and MA 30mg participants rated 12 subjective drug effects on a visual analog scale (VAS).
RESULTS:
As demonstrated by statistically-significant ibudilast×MA condition interactions (p<.05), ibudilast reduced several MA-related subjective effects including High, Effect (i.e., any drug effect), Good, Stimulated and Like. The ibudilast-related reductions were most pronounced in the MA 30mg infusions, with ibudilast 100mg significantly reducing Effect (97.5% CI [-12.54, -2.27]), High (97.5% CI [-12.01, -1.65]), and Good (97.5% CI [-11.20, -0.21]), compared to placebo.
CONCLUSIONS:
Ibudilast appeared to reduce reward-related subjective effects of MA in this early-stage study, possibly due to altering the processes of neuroinflammation involved in MA reward. Given this novel mechanism of action and the absence of an efficacious medication for MA dependence, ibudilast warrants further study to evaluate its clinical efficacy.
https://www.ncbi.nlm.nih.gov/pubmed/25975386
Addict Biol. 2016 Jul;21(4):895-903. doi: 10.1111/adb.12261. Epub 2015 May 14.
The effects of ibudilast, a glial activation inhibitor, on opioid withdrawal symptoms in opioid-dependent volunteers.
Cooper ZD1, Johnson KW1, Pavlicova M2, Glass A3, Vosburg SK1, Sullivan MA1, Manubay JM1, Martinez DM1, Jones JD1, Saccone PA1, Comer SD1.
Author information
Abstract
Glial activation is hypothesized to contribute directly to opioid withdrawal. This study investigated the dose-dependent effects of a glial cell modulator, ibudilast, on withdrawal symptoms in opioid-dependent volunteers after abrupt discontinuation of morphine administration. Non-treatment-seeking heroin-dependent volunteers (n = 31) completed the in-patient, double-blind, placebo-controlled, within-subject and between-group study. Volunteers were maintained on morphine (30 mg, QID) for 14 days and placebo (0 mg, QID) for the last 7 days of the 3-week study. Volunteers also received placebo (0 mg, PO, BID) capsules on days 1-7. On days 8-21, volunteers were randomized to receive ibudilast (20 or 40 mg, PO, BID) or placebo capsules. Subjective and clinical ratings of withdrawal symptoms were completed daily using daily using the Subjective Opioid Withdrawal Scale (SOWS) and Clinical Opioid Withdrawal Scale (COWS). Medication side effects were also monitored. Relative to the first 2 weeks, all groups exhibited withdrawal during the third week as assessed by the SOWS and COWS (P ≤ 0.0001). Although overall SOWS scores did not differ between groups, exploratory analyses pooling the two ibudilast groups demonstrated that they had lower ratings of withdrawal symptoms on SOWS items (‘anxious,’ ‘perspiring,’ ‘restless,’ ‘stomach cramps’) during detoxification relative to the placebo group. Ibudilast was well tolerated; no serious adverse events occurred during the study. Pharmacological modulation of glial activity with ibudilast decreased some subjective ratings of opioid withdrawal symptoms. These exploratory findings are the first to demonstrate the potential clinical utility of glial modulators for treating opioid withdrawal in humans.
https://www.ncbi.nlm.nih.gov/pubmed/23085301
J Stroke Cerebrovasc Dis. 2014 Jan;23(1):51-5. doi: 10.1016/j.jstrokecerebrovasdis.2012.09.007. Epub 2012 Oct 22.
Effect of ibudilast on the reciprocal inhibitory visual-vestibular interaction closely related to dizziness after cerebral ischemia.
Inoue N1, Fukuda S2, Inada T2, Sameshima E2, Tokushima Y2, Harada M2.
Author information
Abstract
BACKGROUND:
Many patients with chronic cerebrovascular diseases suffer dizziness. Our earlier findings suggested that prolonged terms of dizziness episodes may decrease the regional cerebral blood flow (CBF) in the occipital visual cortex via a remote effect from the vestibular cortex.
METHODS:
We studied 9 patients who suffered episodes of dizziness since the onset of chronic cerebral ischemia. Their at-rest CBF was measured at entry into the study and approximately 3 months after the start of ibudilast therapy when all patients reported the resolution of dizziness.
RESULTS:
After 3 months of ibudilast their at-rest CBF was significantly increased in the left occipital lobe (P = .02). CBF after acetazolamide (ACZ) loading was significantly increased in the bilateral occipital lobes (right, P = .049; left, P = .02) and in the bilateral parieto-insular vestibular cortex (PIVC; right and left, P = .02). There were no significant CBF changes in any other areas.
CONCLUSIONS:
Our findings indicate that the occipital cortex and PIVC were implicated in their dizziness after cerebral ischemia. We discuss the underlying mechanism(s) and the relationship between dizziness and reciprocal inhibitory visual-vestibular interactions.
https://www.ncbi.nlm.nih.gov/pubmed/19629961
Biomed Chromatogr. 2010 Mar;24(3):324-8. doi: 10.1002/bmc.1293.
Determination of ibudilast in human serum by high-performance liquid chromatography for pharmacokinetic study.
Yoon H1, Cho HY, Lee YB.
Author information
Abstract
A simple, accurate, precise and cost effective reversed-phase HPLC method was developed to determine the concentration of ibudilast in human serum. Ibudilast and an internal standard, butyl 4-hydroxybenzoate, were extracted by liquid-liquid extraction with methyl tert-butyl ether. HPLC analysis was carried out under the following conditions: a Luna C(18)(2) 5 microm column, a mobile phase of acetonitrile-0.02% phosphoric acid (50 : 50, v/v, adjusted to pH 6.0 with triethylamine) and a UV detector at 319 nm. The chromatograms showed good resolution and sensitivity as well as no interference from the human serum. The calibration curves were linear over the concentration range, 1-100 ng/mL, for serum with correlation coefficients >0.999. The intra- and inter-day assay precision as well as the accuracy fulfilled the international requirements. The mean absolute recovery for human serum was 101.7 +/- 6.1%. The lower limit of quantitation in human serum was 1 ng/mL, which is sensitive enough for pharmacokinetic studies. Stability studies revealed that ibudilast in human serum was stable during storage as well as during the assay procedure. This method was applied successfully to an examination of the pharmacokinetics of ibudilast in human subjects following a single oral dose of an ibudilast (10 mg) capsule.
https://www.ncbi.nlm.nih.gov/pubmed/19452709
Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2009 Jan;23(2):63-6.
Clinical research of Ibudilast on treating the steroid resistant allergic rhinitis.
Luo H1, Tao Z, Yan N, Liang J, Wang P, Wang J.
Author information
Abstract
OBJECTIVE:
To compare the efficacy and safety of histamine H1 receptor antagonist loratadine with Leukotriene receptor antagonist Ibudilast in steroid resistant allergic rhinitis in a randomized controlled clinical trial.
METHOD:
Thirty-five cases were treated by Ibudilast, and 34 cases by loratadine. Score system was used to compare the therapeutic effect of these two drugs on clinical symptoms and signs.
RESULT:
Ibudilast shows a better curative effect than loratadine in the improvement of the total scores on clinical symptom and signs(P<0.05). Scores of symptoms and signs in Ibudilast group after 3, 7, 14 days decreased significantly by means of square analysis of single factor (P<0.01). No complication was observed.
CONCLUSION:
Ibudilast can effectively alleviate the clinical symptoms and signs of steroid resistant allergic rhinitis with confirmed efficacy and safety, thus is recommended in steroid resistant allergic rhinitis. Increased doses or prolonged treatment of steroid is inappropriate.
https://www.ncbi.nlm.nih.gov/pubmed/19032723
Br J Clin Pharmacol. 2008 Dec;66(6):792-801. doi: 10.1111/j.1365-2125.2008.03270.x.
Ibudilast in healthy volunteers: safety, tolerability and pharmacokinetics with single and multiple doses.
Rolan P1, Gibbons JA, He L, Chang E, Jones D, Gross MI, Davidson JB, Sanftner LM, Johnson KW.
Author information
Abstract
AIMS:
To investigate the safety, tolerability and pharmacokinetics (PK) of ibudilast after a single-dose and a multiple-dose regimen.
METHODS:
Healthy adult male (n = 9) and female (n = 9) volunteers were evaluated over a 17-day stay in a Phase 1 unit. Subjects were randomized 1 : 3 to either oral placebo or ibudilast at 30-mg single administration followed by 14 days of 30 mg b.i.d. Complete safety analyses were performed and, for PK, plasma and urine samples were analysed for ibudilast and its major metabolite.
RESULTS:
Ibudilast was generally well tolerated. No serious adverse events occurred. Treatment-related adverse events included hyperhidrosis, headache and nausea. Two subjects discontinued after a few days at 30 mg b.i.d. because of vomiting. Although samples sizes were too small to rule out a sex difference, PK were similar in men and women. The mean half-life for ibudilast was 19 h and median T(max) was 4-6 h. Mean (SD) steady-state plasma C(max) and AUC(0-24) were 60 (25) ng ml(-1) and 1004 (303) ng h ml(-1), respectively. Plasma levels of 6,7- dihydrodiol-ibudilast were approximately 30% of the parent.
CONCLUSIONS:
Ibudilast is generally well tolerated in healthy adults when given as a single oral dose of 30 mg followed by 30 mg b.i.d. (60 mg day(-1)) for 14 days. Plasma PK reached steady state within 2 days of starting the b.i.d. regimen. Exposure to ibudilast was achieved of a magnitude comparable to that associated with efficacy in rat chronic pain models.
https://www.ncbi.nlm.nih.gov/pubmed/18677969
Arzneimittelforschung. 2008;58(6):277-82. doi: 10.1055/s-0031-1296507.
Effect of ibudilast on non-specific symptoms in patients with chronic cerebral ischemia. Analysis of cerebral blood flow.
Inoue N1, Harada M.
Author information
Abstract
BACKGROUND AND PURPOSE:
Many patients with chronic cerebrovascular disease complain that dizziness and depression negatively affect their daily lives. In these patients, ibudilast (CAS 50847-11-5) reportedly ameliorated dizziness. The efficacy of ibudilast was investigated and its effect on the cerebral blood flow (CBF) was recorded.
METHODS:
The study population consisted of 11 patients (male and female) with chronic cerebrovascular disease complaining of dizziness or depression. They received 30 mg of ibudilast orally per day. The grade of vertigo and depression at entry into this study and 2 and 6 months after the start of therapy was recorded. Their depressive state was scored with the Japan Stroke Scale-Depression Scale (JSS-D) and their cerebral blood flow (CBF) was measured before and approximately 3 months after the start of ibudilast therapy.
RESULTS:
At 6 months after the start of ibudilast therapy, all patients reported the resolution of dizziness; of the 6 patients with depression at entry, all experienced significant improvement. At 3 months, the CBF was significantly increased in the right frontal (p = 0.019) and occipital cortex (p = 0.004) with no significant changes in the cerebellar folia, subcortical gray matter (striatum and thalamus), and other cerebral cortices in the right cerebral hemisphere. There were no significant CBF changes in any areas of the left cerebral hemisphere.
CONCLUSION:
In patients treated with ibudilast, the amelioration of dizziness and depression was accompanied by a CBF increase in the right frontal and occipital cortices. These findings suggest that the right frontal and occipital cortices may be related to their dizziness and depression.
https://www.ncbi.nlm.nih.gov/pubmed/16358931
J UOEH. 2005 Dec 1;27(4):377-83.
Clinical application of ibudilast for elder patients with chronic subdural hematoma.
Ohta H1, Genmoto T, Akiba D, Urasaki E, Yokota A.
Author information
Abstract
Ibudilast, an antagonist of platelet-activating factor receptors, was administered to patients with chronic subdural hematoma (CSDH) to assess its effectiveness in preventing recurrence. The remaining volumes of subdural hematomas on brain computed tomography were measured approximately 1-2 months after using ibudilast. The hematomas were significantly smaller and there was no recurrence. Ibudilast administration may be useful in the prevention of recurrence of CSDH.
https://www.ncbi.nlm.nih.gov/pubmed/15471363
Mult Scler. 2004 Oct;10(5):494-8.
Ibudilast, a nonselective phosphodiesterase inhibitor, regulates Th1/Th2 balance and NKT cell subset in multiple sclerosis.
Feng J1, Misu T, Fujihara K, Sakoda S, Nakatsuji Y, Fukaura H, Kikuchi S, Tashiro K, Suzumura A, Ishii N, Sugamura K, Nakashima I, Itoyama Y.
Author information
Abstract
We investigated the immunoregulatory effects of ibudilast, a nonselective phosphodiesterase inhibitor, at a clinically applicable dose (60 mg/day p.o. for four weeks) in multiple sclerosis (MS) patients. Sensitive real-time PCR for quantifying cytokine mRNA in the blood CD4+ cells revealed that the ibudilast monotherapy significantly reduced tumour necrosis factor-alpha and interferon (IFN)-gamma mRNA and the IFN-gamma/interleukin-4 mRNA ratio, suggesting a shift in the cytokine profile from Th1 toward Th2 dominancy. In a flow cytometric analysis, natural killer T cells, which have been reported to relate to Th2 responses in MS and its animal model (experimental autoimmune encephalomyelitis), increased significantly after the therapy. None of the significant immunological changes were seen in healthy subjects or untreated MS patients. Ibudilast may be a promising therapy for MS and its clinical effects warrant further study.
https://www.ncbi.nlm.nih.gov/pubmed/7639416
Angiology. 1995 Aug;46(8):699-703.
Efficacy of Ibudilast on lower limb circulation of diabetic patients with minimally impaired baseline flow: a study using color Doppler ultrasonography and laser Doppler flowmetry.
Sone H1, Okuda Y, Asakura Y, Asano M, Mizutani M, Bannai C, Yamashita K.
Author information
Abstract
Ibudilast is a prostacyclin-mediated vasodilator and antiplatelet agent. The hemodynamic effects of ibudilast were evaluated in 41 patients with non-insulin-dependent diabetes mellitus by means of two-dimensional Doppler ultrasonography and laser Doppler blood flowmetry. Before and one hour after oral administration of ibudilast (10 mg), or elastase (1800 U) as a control, the cross-sectional area (CSA) of the dorsal pedis artery, its blood flow index (BFI), and dermal microcirculatory blood volume (MBV) were measured. In the ibudilast group, all of the parameters (CSA, BFI, and MBV) significantly increased as compared with the elastase group. These data suggest that ibudilast is effective in ameliorating diabetic macroangiopathy and microangiopathy of the lower limbs.
https://www.ncbi.nlm.nih.gov/pubmed/8103582
Neurol Res. 1993 Jun;15(3):169-73.
Pharmacological effects of ibudilast on cerebral circulation: a PET study.
Fukuyama H1, Kimura J, Yamaguchi S, Yamauchi H, Ogawa M, Doi T, Yonekura Y, Konishi J.
Author information
Abstract
To evaluate the pharmacological effects of ibudilast on cerebral circulation, we examined 5 ischaemic stroke patients, using positron emission tomography, and we measured cerebral blood flow (CBF). Ibudilast loading per os led to a remarkable increase in CBF after 30 min. This CBF elevation was observed in the lesion side as well as in the nonlesion hemisphere. Therefore, we can confirm that ibudilast ameliorates the chronic low perfusion state of CBF in the ischaemic stroke patient.
https://www.ncbi.nlm.nih.gov/pubmed/8356168
Seishin Shinkeigaku Zasshi. 1993;95(5):392-416.
A quantitative pharmaco-EEG study on psychotropic properties of cerebral metabolic enhancers: comparison between young and elderly healthy volunteers.
Nobuhara K1.
Author information
Abstract
In order to investigate psychotropic properties of cerebral metabolic enhancers (CMEs), the author carried out two identical quantitative pharmaco-EEG studies in different age groups of healthy volunteers; the young group (group-Y) consisted of six males between the ages of 21-26 years and elderly group (group-E) consisted of six males between the ages of 60-66 years. The drugs tested were five CMEs, dihydroergotoxine mesylate (DHE), propentofylline (PPF), nicergoline (NCG), lisuride maleate (LIS) and ibudilast (IDL). Each volunteer received either of the five test drugs or inert placebo in six one-day weekly sessions’ according to single-blind, randomized crossover design. In each session, a single oral dose, equivalent to the clinically recommended daily dose, of either drug or placebo was administered and EEGs were recorded before and 1.3 and 6 hours after the drug administration. Firstly, the background EEGs before the drug administration were compared between the two groups. Group-E showed less slow activities and more alpha and fast activities than group -Y. This difference in background EEG profiles between two groups are considered to be due to physiological aging process. Secondly, drug effects on EEGs in two groups were compared. There were discrepancies in drug-induced EEG changes between the groups. In group-Y, any of the five tested CMEs did not induce EEG changes that were significantly different from placebo, whereas, in group-E, drug-induced EEG changes were more apparent. In group-E, DHE and PPF induced similar EEG changes, which were characterized by a decrease of alpha activity associated with marked decreases of slow and fast activities, the EEG profile similar to thymoleptics with sedative effects. LIS and IDL induced a decrease of alpha activity and an increase of fast activities, the profile close to thymoleptics with mood-elevating (stimulant) effects. NCG induced an increase of slow activities, the profile close to central depressants. These results in this study coincided with the experimental and subjective classification of the clinical effects of CMEs. Further analysis of EEG profiles based on principal component analysis indicated that there were two major components in background EEGs. There were discrepancies in the response to CMEs between two groups. In group-Y, CME-induced changes were seen mainly in the second principal component, while in group-E, the changes were seen mainly in the first principal component. These results suggested CMEs provoked thymoleptic effects by affecting the essential component of the EEG basic rhythm.(ABSTRACT TRUNCATED AT 400 WORDS)
https://www.ncbi.nlm.nih.gov/pubmed/8476664
No To Shinkei. 1993 Feb;45(2):139-42.
SPECT evaluation of effect of cerebral vasodilator by the subtraction method using Tc-99m HMPAO.
Sugiyama Y1, Haba K, Yokoyama K.
Author information
Abstract
The effects of cerebral vasodilator are generally evaluated by observing whether the clinical improvement is applicable after the period of the drug administration. This report describes a novel approach to the evaluation for the effect of the drug using Tc-99m HMPAO. Consecutive brain Tc-99m HMPAO studies before and after a cerebral vasodilator, Ibudilast, administration were performed within 5 hours on 10 patients with cerebral infarction at chronic state. Five patients showed increased perfusion nearby the affected vascular territories after the po administration of Ibudilast. Significant changes in the brain perfusion pattern were determined using an image subtraction technique. This consecutive Tc-99m HMPAO subtraction SPECT technique seems to be useful for evaluating the therapeutic effect of cerebral vasodilator. This method can be performed within a short period of time, safely and sensibly.
http://www.ncbi.nlm.nih.gov/pubmed/1634449
J Asthma. 1992;29(4):245-52.
Effect of ibudilast: a novel antiasthmatic agent, on airway hypersensitivity in bronchial asthma.
Kawasaki A1, Hoshino K, Osaki R, Mizushima Y, Yano S.
Author information
Abstract
Ibudilast, a unique agent with vasodilating and antiallergic actions, was studied in 13 asthmatics for its effect on airway hypersensitivity to histamine inhalation. The PC20 values improved significantly from 355.6 to 620.5 micrograms/ml at 3 months and further to 731.4 micrograms/ml at 6 months following the initial treatment with ibudilast (20 mg twice daily orally). In addition, the severity of the attacks decreased significantly. Improvements in the PC20 and asthmatic symptoms also were observed in the disodium chromoglycate group, but these were equal to or lesser than those in the ibudilast group. No improvement was observed in the untreated control group. These results suggest that ibudilast would be an effective agent for improving nonspecific airway hypersensitivity in asthmatics.
http://www.ncbi.nlm.nih.gov/pubmed/1436980
Ophthalmic Res. 1992;24(4):197-202.
Ibudilast may improve retinal circulation in patients with diabetes mellitus.
Suzuki R1, Sugihara I, Ishibashi T, Kurimoto S.
Author information
Abstract
Ibudilast selectively vasodilates cerebral vessels without reducing blood pressure. We investigated the effect of the drug on retinal circulation in 8 patients with diabetes mellitus, using the video-densitometric image analysis of fluorescein angiography. We compared the build up time, the time constant of washout rate and the mean circulation time (MCT) before and after oral therapy with ibudilast. After 2 weeks of daily administration (30 mg), MCT was shortened significantly (4.2 +/- 2.8 vs. 3.0 +/- 1.6 s, p = 0.0215). Since retinal circulation in patients with diabetes mellitus was improved by ibudilast, the drug may be useful to treat disorders such as diabetic retinopathy.

3 Comments
Leave your reply.